The “About This Technology” section aims to bring our readers up to speed on the concepts and terms behind modern video otoscope technology. Additionally, this section looks at what it takes to get this technology past the assessment phase and into the hands of clinicians, with a discussion on planning to deploy and support this technology. Also included are resources and standards related to video otoscopes.
Video Otoscopes – Technology Overview
Video otoscopes have played a useful role in telemedicine programs for decades, with practical applications in both store-and-forward and videoconferencing. Technological advances over the years have resulted in different approaches to optics, lighting, image sensors, device form factor, and use cases, resulting in a diverse group of products with widely varying levels of performance.
The market has undergone changes in recent years as imaging technology continues to reduce in cost, resulting in high-resolution devices being available at affordable prices. However, video quality for ear imaging is more complicated than simply improving the resolution of a sensor, and some new high-definition cameras may not provide the image quality desired by all clinicians. For this reason, TTAC strongly recommends a robust assessment process when choosing an otoscope for your telehealth practice.
Three notable shifts occurred in the video otoscope market over the past decade:
- The majority of devices now come with a USB-based connection, which simplifies the process of attaching devices to desktop- and laptop-based videoconferencing platforms while making it harder to connect with some dedicated hardware-based videoconferencing systems.
- Affordable, USB-based otoscope devices are now widely available, though these devices may sometimes suffer from low image quality and ease of use challenges.
- More vendors offer multi-purpose examination cameras, offering otoscope attachments in addition to general examination, dermatology, and other lenses.
Understanding how these devices work is an important step in selecting the right otoscope for your telehealth program. Figures 1 through 3 show three different styles of otoscope. The first image shows a light-box-based otoscope with a rod lens, while the second image shows a model without a lens, and the third shows a multipurpose camera with an otoscope attachment. Additional information is included beneath each image.
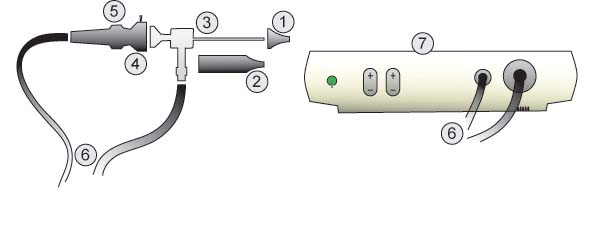
Parts:
- Speculum
- Barrel
- Rod Lens
- Coupler
- Focus Ring
- Cables
- Light Box
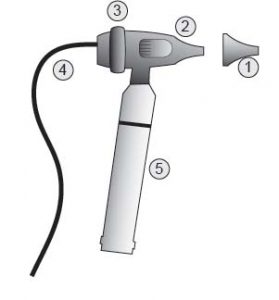
Image 2 – Video Otoscope with USB
Parts:
- Speculum
- Camera Body
- Focus Ring
- Cables
- Battery
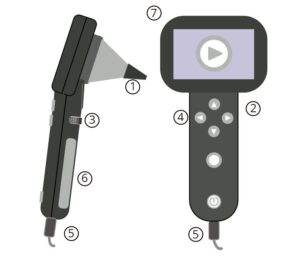
Image 3 – Multipurpose Camera with Otoscope Lens
Parts:
- Speculum
- Camera Body
- Focus Ring (some models)
- User Controls
- Cables
- Screen (some models)
Note that there are variations of these basic components in different manufacturers’ products. These possible differences will be discussed as each part of the otoscope is examined in more detail.
The speculum is typically the only surface of the otoscope that will contact the patient when imaging the ear canal and tympanic membrane. There are several key things to keep in mind when considering specula:
- The exact size and model of speculum that an otoscope can accept will vary by manufacturer, which means your clinic may require multiple models of specula if your standard otoscope specula do not fit on your video otoscope. It is advisable to check with each manufacturer to see if your organization’s existing specula may be used with the video otoscopes, or if proprietary specula can be obtained in adequate numbers for your program.
- Some devices utilize more expensive, washable specula or have a cleanable tip built into the device, which require additional cleaning processes that are not required for models disposable specula.
- Not all devices offer pediatric specula, which may not be acceptable depending on your patient population.
A rod lens is a long, slender lens that generally extends to the tip of the specula. This generally produces an image with a wide field of view and less obstruction by the specula, but introduces challenges with lighting, distortion, and resolution that manufacturers may address with varying degrees of success. The rod lens generally is surrounded by an outer fiber optic ring, which provides illumination while imaging.
The barrel provides some level of protection to the exposed rod lenses and provides a way to mount the speculum. Some providers may choose to use the rod lens without a barrel, resulting in a very narrow device and the inability to use the device with a speculum. Using the barrel or not can be a matter of personal preference; some clinicians find it easier to hold an otoscope with a barrel, while others prefer to image entirely without a barrel and specula. Clinicians should use extreme caution to protect patient safety if choosing not to use the barrel and/or specula features of a video otoscope.
The coupler, which is present on otoscopes with rod lenses as well as multipurpose exam cameras, is used to attach the lens to the camera body and imaging sensor. It is important to consider the alignment of the lens with the camera when using a rod lens; ensure continued proper alignment by securing any locking mechanisms once the initial alignment is verified to be correct or the imaging sensor may rotate and the orientation of the picture may be out of alignment. All multipurpose exam cameras and some otoscopes with rod lenses include a marker or icon on the coupler or camera body that indicates proper alignment between the lens and camera body. Note that it may be possible to use barrel and rod lens assemblies from one manufacturer with the camera body and coupler of another, and TTAC testing found that combining different lenses and camera bodies may result in superior images. Note this may not be a supported configuration with the manufacturer and should be verified on a case-by-case basis should your organization be interested in using existing rod lenses with new video otoscopes.
Some otoscopes forego a barrel and lens assembly, instead incorporating an imaging sensor with a series of surface-mounted LEDs for illumination. These devices require a specula in order to image the tympanic membrane, but may be used without a specula for intraoral or external imaging.
Camera bodies come in many different shapes and styles. In general, the camera bodies will contain an imaging sensor and built-in light source or fiber optic connections that transmit light from external sources.
Most otoscopes will have controls to adjust device settings. Common controls include a focus ring, brightness controls, and image capture. Some devices may also have controls to power the device on or off.
The focus ring allows the user to adjust the focal point of the lens, or how far away an object can be while staying clear and sharp. Some products do not have a focus ring, and instead maintain a fixed focus that is pre-set to allow good imaging of the tympanic membrane. Still other devices will utilize auto-focus functionality, removing the need to manual focus the device. Some products support a broad focal range, while others are much more limited. There are many possible factors in how well a device can be focused. The subject of focus is an important one with video otoscopes.
The majority of devices will have one or more cables coming from the camera body. These cables can serve many purposes:
- Provide light from a light box or LED light source
- Transmit video or data to a PC, or external video input (such as a monitor or video endpoint.
- Provide power to run or recharge the device
Most devices will use at least one USB cable, which can provide power, data, and video transmission. Video otoscopes frequently transmit video over USB, but a number of manufactures still support a range of outputs, including S-Video, Composite, HDMI, mini-HDMI, and SDI. Note: while most computer cables are resilient to some level of physical strain, extra attention should be paid to crushing and crimping if an otoscope depends on fiber optic cables. Fiber optic cables are composed of many long strands of glass, and responsible for the direct transmission of light from a light box. Damage to these fiber optic cables can result in decreased light output and image quality. Only devices with have a separate light box, with the illumination source in the box, utilize a fiber optic cable.
Common Video Otoscope Problems and Key Imaging Concepts
Video otoscopes are prone to several problems that can make capturing quality images challenging. Issues around light control, focusing, and distortion are fairly common, as are overall problems with image quality. The root causes of these problems are varied. In some instances, algorithms and embedded controls are responsible for the problems (and correction of them), while others are caused by the hardware itself.
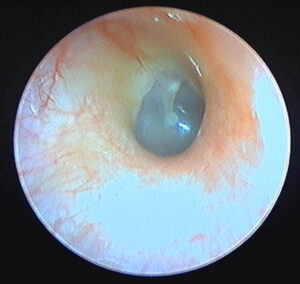
Blooming
The problem of blooming results from the inability of the imaging sensor to compensate for brightly-illuminated regions of a subject, with highly-reflective surfaces being especially prone to causing blooming in an image. This “over saturation” is visible in the image as a white highlight, and the blooming extends beyond the single point of highlight, often “bleeding out” or “blowing out” details in the image.
Otoscopes implement gain control to mitigate this problem, though they use different techniques and terminology for this function. Some devices will automatically adjust the , while others require manual adjustments. Otoscopes also often allow for light intensity to be changed, which can also assist in reducing blooming. Testing revealed that each device has a slight learning curve in order to capture the best image, with a balance between light intensity and gain settings.
Focusing
Being able to properly focus an otoscope is one of the most critical elements in capturing a good image. Unfortunately, this process is not always easily done with all otoscopes as they may have controls that are difficult to operate or which are overly responsive and challenging to “dial in” precisely.
Focusing challenges can be exacerbated if the otoscope has a limited depth of field. Depth of field relates to how much “depth” in an image remains in focus; in extreme cases a shallow depth of field will mean that the part in focus may be sharp and clear but any parts of the anatomy a millimeter nearer to or further from the lens may be blurry. Additionally, these shallower depths-of-field can be problematic, as it means that miniscule movements of the probe will move the subject out of the area of focus and result in a blurry image. For other products, the depth of field may extend for over a centimeter, which may make the device easier to use.
The physical act of focusing becomes much more important as the depth of field decreases. A balance needs to be struck with the focus ring, weighing between being easily changed while imaging and being capable of holding focus once set. In general, otoscopes either need to have an easily accessible mechanism for focusing during an exam, or the focus needs to be set outside of the ear prior to beginning the exam.
Some devices suffer from an issue that is loosely related to focusing. On products with surface-mounted LEDs located on the same plane as the lens, focusing on surfaces near to the end of the specula can result in a vignette, halo or light around the edge of the image.
Field-of-View
When imaging a tympanic membrane, a typical clinician will want to view as much of the surface of the TM in a single image as possible, which depends in large part on the device’s field of view. Field of view refers to how much of a subject is visible from the lens, often discussed in terms of degrees. Devices with a narrow field of view will only be able to see a portion of the tympanic membrane, requiring multiple still images or a moving live video feed to show the complete surface.
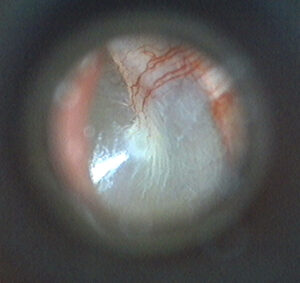
Devices with a very wide field of view are prone to barrel distortion. This distortion can make an image appear to “bulge” out towards the viewer, and may add apparent depth to an image that isn’t actually there. Extreme cases of this can make an image appear to be spherical.

Image Quality – Color and Detail
Accurate, life-like colors are an important element of diagnosing ear disease with a video otoscope. Inaccurate colors may result in normal ear appearing red and irritated, pale and cadaverous, or jaundiced. “White balancing,” or establishing what true white is in an image, is the term often used to describe the process of setting an otoscope to capture true-color images. Otoscope manufacturers typically take one of two approaches to white balancing, with some automatically, dynamically setting the white balance through algorithms in the device, and others requiring a manual step at each use.
The accuracy of automatic white balancing varies fairly widely across manufacturers. Some devices will drastically change the white balance with minor movements of the probe, resulting in noticeable variations in color between very similar images.
Manual white balancing typically requires that the user direct the probe at a white sheet of paper while pressing a button. The otoscope will then take a reading of the incoming image and adjust the interpretation of the color to set a new white balance. While generally seen as a more reliable method for white balancing, it can be problematic if users forget to white balance, set the balance against something that is not truly white, or if the device is used to capture images at a distance significantly greater than or less than was used during white balancing.
Issues in color can also arise with contrast, brightness and darkness, saturation, and hue. The causes for these problems vary, but are often tied to how the image is processed by the internal components of the otoscope. As such, users generally have limited ability to manually change those settings. Additionally, individual computer monitors can display colors differently, rendering even the most color-accurate video otoscope image less useful. Note that it is possible to properly calibrate a computer monitor, and this process is highly recommended when performing both evaluation o otoscope devices as well as when performing clinical diagnosis.
Image detail can be impacted by several factors, including the resolution of the imaging sensor, the quality of the lighting, the sensitivity of the imaging sensor to light, and the reaction of some lenses to the humid environments of the ear, nose, and throat. Under-illuminated images often appear grainy. It is important to ensure that the otoscope’s light source is set at the correct level when imaging. Some otoscopes combat the “clouding” issue of imaging in a humid environment by providing support for insufflation bulbs that allow air to be directed to the tip of the probe.
Video Otoscopes – Deployment and Support
The deployment of equipment can be made significantly easier if proper planning activities take place prior to shipping otoscopes out to sites. Cutting corners before sending devices to the field can cause your program to incur costs later on as support staff are forced to resolve problems for unhappy and frustrated clinicians. It is critical to take time for planning prior to placing orders for equipment.
Planning
Think through how the end sites will be supported by your organization. Regardless of whether individual sites will buy equipment individually or if all purchases will be made in a single bulk order, it is important to establish a baseline of standardized parts, required consumables, and intended support systems.
- How many video otoscopes need to go to each site? Will every end site have their own camera, or will it vary on an organization-by-organization basis?
- Will sites need to purchase their own specula for their otoscopes? Are they the same specula as used on existing otoscopes? How many will they need?
- If an otoscope breaks, will it be shipped back to a central program office for replacement and warranty work, or will the individual sites be responsible for contacting the vendor or manufacturer?
- If video otoscopes wear out or break, will the program office have the capacity to provide a replacement device? How many replacement otoscopes can you afford to have in stock as spare devices?
- Will the program office establish standard settings for the otoscopes before sending them to the sites? This can prevent configuration problems after deployment, but has an up-front cost to configure each otoscope and requires space to receive, configure, store, and ship the devices. If individual sites are purchasing their own otoscopes, will a guide be created to explain the preferred settings?
Purchasing
With planning out of the way, it is time to make purchasing decisions. Depending on the answers to the above questions, this may be as simple as telling the sites to find the correct make and model of otoscope on their own. There are often benefits to purchasing devices in bulk, and there may be a way to get discounts if purchasing a high-enough quantity of video otoscopes. Try to establish a relationship with the vendors and resellers so that they understand your program’s needs. This is true now more than ever as supply chain disruptions make procuring equipment more challenging.
A healthy relationship with an otoscope manufacturer or reseller can be beneficial over the life of a telehealth program. Aside from possible reduced unit costs, it may be possible to discuss extended warranties for sites or to set up a service plan that meets your organizational needs. It may also be possible to help influence future designs in otoscopes, as the manufacturers may listen to large customers in what is still a relatively small field.
Deployment
Talking with the end sites is critical when planning a deployment. If key information is not passed along, equipment may sit in a warehouse or loading dock, or may go to the wrong location. Getting the end sites involved in deployment planning early can make sure everyone knows what to expect, and can mitigate feelings that the otoscopes are just being delivered to sites without a clear implementation plan.
If the video otoscopes are being received by a central program office, temporary warehouse or storage space may be needed, depending on the number of otoscopes being received. Being able to dedicate space to the receiving and staging of video otoscopes can be beneficial, as it keeps other activities from impeding various deployment processes.
Significantly more staging room may be required if the video otoscopes will be tested or configured prior to deployment, with inventory management controls in place to ensure that devices are all successfully configured and not misplaced. Clear areas should be established for otoscopes that are ready to ship, and devices that are awaiting configuration. Ensure that all necessary components of the video otoscopes make it back into the box, as well as any additional consumables, connectors, or educational products that will be deployed with otoscope.
Support
Depending on service level agreements with the end sites, support may or may not fall under the purview of a central office. Regardless of the level of support offered to end sites, a mechanism for reporting problems should be available. If sites are having issues with the otoscope, it can be important to know what the problem is and where it is located. Is the video otoscope too difficult to use? Perhaps this means that additional training or support materials are required. Are certain populations of patients finding the otoscope to be uncomfortable? Perhaps larger or smaller specula are needed at the sites. Are users reporting that it is challenging to capture clear images? This may require additional end-user training or device maintenance. Is the otoscope wearing out? Are light sources regularly failing? Widespread reports of this may be a sign that the otoscopes are in need of maintenance.
In addition to listening to your end sites, it is also good to check in with the providers who are reviewing images or providing consults. Feedback from these sources can identify trends in poor quality images, poor end user training, or possible equipment failures. This information can be used to identify issues with equipment, procedures, and training support.
Work with the sites when the video otoscopes first arrive. Ask if there are any problems or issues with the devices, and if possible request that they send a test images or join a quick video call to validate the output from the devices. This allows a central project office to ensure that the otoscopes have been received, unpacked, and used by the end sites. Any problems can be identified immediately and resolved quickly.
Video Otoscopes – Resources
Terms: Otolaryngology, Telemedicine, Evaluation, Telehealth, Otoscope
Barriga F, Schwartz R, hayden G. Adequate Illumination for otoscopy. Variations due to power source, blub and head and speculum design. Am J Dis Child 1986;140L1237-1240.
Birkmire-Peters, D., Peters, L., & Whitaker, L. (1999). A usability evaluation for telemedicine medical equipment. Telemedicine Journal: The Official Journal Of The American Telemedicine Association, 5(2), 209-212.
Burgess, L., Holtel, M., Syms, M., Birkmire-Peters, D., Peters, L., & Mashima, P. (1999). Overview of telemedicine applications for otolaryngology. Laryngoscope, 109(9), 1433-1437.
Eikelboom RH, Atlas MD, Mbao MN, Gallop M. Teleotology: planning, design, development and implementation. J Telemed Telecare 2002;8(Suppl 3):S3:14–17.
Eikelboom, R. H., Weber, S., Atlas, M. D., Quan, D., Mbao, M. N., & Gallop, M. A. (2003). A tele-otology course for primary care providers. Journal of Telemedicine & Telecare, 919-22.
Goldenberg, D., & Wenig, B. (2002). Telemedicine in otolaryngology. American Journal of Otolaryngology, 23(1), 35-43.
Heneghan, C., Sclafani, A., Stern, J., & Ginsburg, J. (1999). Telemedicine applications in otolaryngology. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society, 18(4), 53-62.
Hofstetter, P., Kokesh, J., Ferguson, A., & Hood, L. (2010). The impact of telehealth on wait time for ENT specialty care. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 16(5), 551-556.
Holtel, M., & Burgess, L. (2002). Telemedicine in otolaryngology. Otolaryngologic Clinics Of North America, 35(6), 1263-1281.
Ibekwe, T., Adeosun, A., & Nwaorgu, O. (2009). Quantitative analysis of tympanic membrane perforation: a simple and reliable method. The Journal Of Laryngology And Otology, 123(1), e2.
Kokesh, J., Ferguson, A., & Patricoski, C. (2010). Preoperative planning for ear surgery using store-and-forward telemedicine. Otolaryngology–Head And Neck Surgery: Official Journal Of American Academy Of Otolaryngology-Head And Neck Surgery, 143(2), 253-257.
Kokesh, J., Ferguson, A., Patricoski, C., & LeMaster, B. (2009). Traveling an audiologist to provide otolaryngology care using store-and-forward telemedicine. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 15(8), 758-763.
Kokesh, J., Ferguson, A., Patricoski, C., Koller, K., Zwack, G., Provost, E., & Holck, P. (2008). Digital images for postsurgical follow-up of tympanostomy tubes in remote Alaska. Otolaryngology–Head And Neck Surgery: Official Journal Of American Academy Of Otolaryngology-Head And Neck Surgery, 139(1), 87-93.
Lundberg, T., Westman, G., Hellstrom, S., & Sandstrom, H. (2008). Digital imaging and telemedicine as a tool for studying inflammatory conditions in the middle ear–evaluation of image quality and agreement between examiners. International Journal Of Pediatric Otorhinolaryngology, 72(1), 73-79.
Mathew N., M., Robert H., E., Marcus D., A., & Mark A., G. (2003). Evaluation of Video-Otoscopes Suitable for Tele-Otology. Telemedicine Journal & E-Health, 9(4), 325-330.
Mbao, M., Eikelboom, R., Atlas, M., & Gallop, M. (2003). Evaluation of video-otoscopes suitable for tele-otology. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 9(4), 325-330.
Melcer, T., Hunsaker, D., Crann, B., Caola, L., & Deniston, W. (2002). A prospective evaluation of ENT telemedicine in remote military populations seeking specialty care. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 8(3), 301-311.
Patricoski C, Kokesh J, Ferguson AS, et al. A comparison of in-person examination and video otoscope imaging for tympanostomy tube follow-up. Telemed J E Health 2003;9: 331–344.
Patricoski, C., Kokesh, J., Ferguson, A., Koller, K., Zwack, G., Provost, E., & Holck, P. (2003). A comparison of in-person examination and video otoscope imaging for tympanostomy tube follow-up. Telemedicine Journal And E-Health: The Official Journal Of The American Telemedicine Association, 9(4), 331-344.
Pepper, J., Jackson, R., Crumley, R., & Wong, B. (2006). Design of a low-cost, USB-compatible, otoscope image-capture system. The Laryngoscope, 116(12), 2224-2226.
Quantitative analysis of tympanic membrane perforation: a simple and reliable method. (2009). Journal of Laryngology & Otology, 123(1), 0.
Sclafani, A., Heneghan, C., Ginsburg, J., Sabini, P., Stern, J., & Dolitsky, J. (1999). Teleconsultation in otolaryngology: live versus store and forward consultations. Otolaryngology-Head & Neck Surgery, 120(1), 62-72.
Smith, A. C., Perry, C., Agnew, J., & Wootton, R. (2006). Accuracy of pre-recorded video images for the assessment of rural indigenous children with ear, nose and throat conditions. Journal of Telemedicine & Telecare, 1276-80.
Smith, A. C., Williams, J., Agnew, J., Sinclair, S., Youngberry, K., & Wootton, R. (2005). Realtime telemedicine for paediatric otolaryngology pre-admission screening. Journal of Telemedicine & Telecare, 11(8), 86-89.
Smith, A., Dowthwaite, S., Agnew, J., & Wootton, R. (2008). Concordance between real-time telemedicine assessments and face-to-face consultations in paediatric otolaryngology. The Medical Journal Of Australia, 188(8), 457-460.
Smith, R. (2002). The digital camera in clinical practice. Otolaryngologic Clinics Of North America, 35(6), 1175-1189.
Ullah R, Gilliland D, Adams D: Otolaryngology consultations by real-time telemedicine. Ulster Medical Journal 2002, 71(1):26-6.
Using the ENT Scope/Video Otoscope. University of Hawaii, 2001.
W.S. Jones, Video otoscopy: bringing otoscopy out of the ‘‘black box’’, Int. J. Pediatr. Otorhinolaryngol. 70 (2006) 1875—1883.
Xu, C. Q., Smith, A. C., Scuffham, P. A., & Wootton, R. (2008). A cost minimisation analysis of a telepaediatric otolaryngology service. BMC Health Services Research, 81-10. doi:10.1186/1472-6963-8-30
